
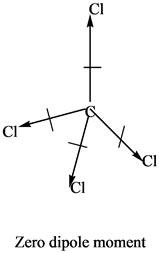
It has the ability to accelerate the burning of combustible material.īefore we dive deeper let’s clear a few terms and other important things as mentioned in the below subtopics.Ĭonclusion How to identify a polar or non-polar molecule? This is a type of colorless, fuming liquid with a pungent odor that is mostly used as a fluorinating agent to produce fluorocarbons and also as an oxidizer in rocket propellant systems and is a very toxic chemical by inhalation. Therefore this molecule is ought to be polar. The molecule contains a central bromine atom which is encompassing a total of five fluorides and forming a lone pair of electrons. So, Is BrF5 Polar or Non-Polar? BrF5 or Bromine Pentafluoride is a polar molecule as the molecular geometry of BrF5 falls out to be square pyramidal with an asymmetric charge distribution concentrating on the central atom. Hence, some of the molecules face this issue in which BrF5 also falls. This is not the end as some molecules even fall somewhere on the spectrum scale between the two classes. Most of the time molecules can easily be declared polar or non-polar whereas, some of them are hard to identify in polarity. When it comes to the polarity of a molecule, a simple question that strikes our mind considering BrF5 or (Bromine Pentafluoride as it is popularly known), is whether it’s a polar or non-polar molecule? As in Chemistry, the two main classes of molecules are divided into polar molecules and non-polar molecules.


 0 kommentar(er)
0 kommentar(er)
